Spectrophotometric Estimation of Phenylephrine Hydrochloride via Oxidative Coupling Reaction with p-Aminobenzophenone
DOI:
https://doi.org/10.48112/bcs.v2i2.455Abstract
 Abstract Views: 356
Abstract Views: 356
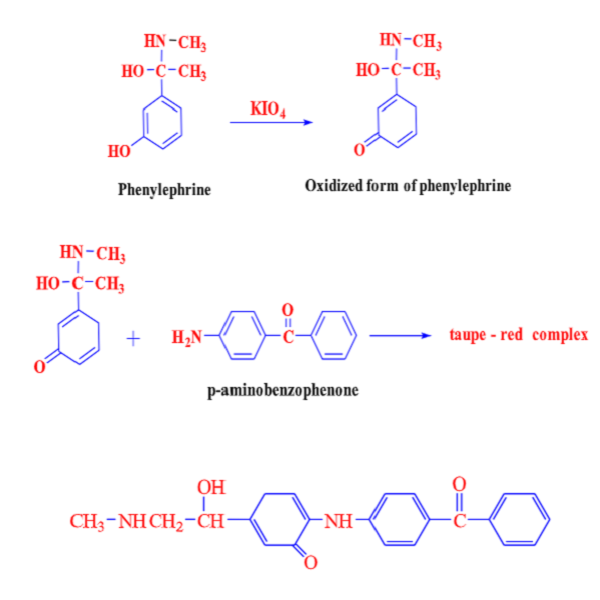
In this research, a rapid, simple and accurate spectrophotometric approach was described for the estimation of phenylephrine hydrochloride in the pure and in its drug forms. The suggested method was based on the oxidative coupling reaction of phenylephrine hydrochloride with p-aminobenzophenone using potassium periodate as an oxidant. A taupe-red dye was formed at room temperature and showed maximum absorption at 512 nm. The linearity of the standard calibration curve was compatible with Beer's law within the concentration range of 2.0-20 μg/mL with a determination coefficient (r2=0.9986). The apparent molar absorptivity and the sensitivity of Sandell's index were calculated and found to be in the values of 0.552x104 L/mol.cm. and 0.0368 μg/cm2, respectively. The nature of the resulting dye has been studied between phenylephrine hydrochloride to p-aminobenzophenoneand and it was equal to 1:1. The limits of detection (LOD) and quantification (LOQ) were estimated and found to be 0.0094 and 0.0313 μg/mL, respectively. A relative standard deviation and a relative error were also calculated and they will be in the range of 0.0715 to 0.0216 and -0.0479% to -0.0145%, respectively. The recommended procedure was applied to assay phenylephrine hydrochloride in drops and injection and no interferences were observed from the common additives found in the drugs.
Keywords:
Oxidative coupling reaction, Phenylephrine hydrochloride, P-Amino benzophenon, SpectrophotometryMetrics
References
Ahmed, A. M. K., Anwar, S. M., & Hattab, A. H. (2020). Spectrophotometric determination of phenylephrine hydrochloride in pharmaceutical preparations by oxidative coupling reaction. Int. J. Drug Delivery Technol., 10, 323-327.
Aljeboree, A. M., & Alshirifi, A. N. (2018). Colorimetric Determination of phenylephrine hydrochloride drug Using 4-Aminoantipyrine: Stability and higher sensitivity. Journal of Pharmaceutical Sciences and Research, 10(7), 1774-1779.
Al-Shaalan, N. H. (2010). Determination of phenylephrine hydrochloride and chlorpheniramine maleate in binary mixture using chemometric-assisted spectrophotometric and high-performance liquid chromatographic-UV methods. Journal of Saudi Chemical Society, 14(1), 15-21. https://doi.org/10.1016/j.jscs.2009.12.004
Alteemi, H. S., & Kadim, K. H. (2020, November). Colorimetric determination of phenylephrine hydrochloride drug by diazotization reaction. In Journal of Physics: Conference Series (Vol. 1664, No. 1, p. 012097). IOP Publishing. http://dx.doi.org/10.1088/1742-6596/1664/1/012097
Al-Uzri, W. A. (2019). Determination of phenylephrine hydrochloride in pharmaceutical preparations using spectrophotometric method. Asian J. Pharm. and Clinical Rese, 12(5), 1-5.
Battu, S., Gandu, V., & Nenavathu, B. P. (2020). Simple spectrophotometric method for estimation of drugs using chloramine-t and indigo caramine dye couple. Asian J Biomed Pharmaceut Sci, 10(69), 19.
Battu, S., Gandu, V., & Nenavathu, B. P. (2020). Simple spectrophotometric method for estimation of drugs using chloramine-t and indigo caramine dye couple. Asian J Biomed Pharmaceut Sci, 10(69), 19.
British Pharmacopaeia (2022). H.M. Stationary Office, London.
Christian, G. D., Dasgupta, P. K., & Schug, K. A. (2013). Analytical chemistry. John Wiley & Sons.
De Levie, R. (1997). Principles of quantitative chemical analysis. McGraw-Hill Science, Engineering & Mathematics.
Dinc Zor, S., Aksu Donmez, O., Ascı, B., & Yarkadas, G. (2017). A novel RP-HPLC method for the simultaneous analysis of some active ingredients in cough–cold syrup formulation. Current Pharmaceutical Analysis, 13(3), 304-313.
Goodman, L. S. (1996). Goodman and Gilman's the pharmacological basis of therapeutics (Vol. 1549, pp. 1361-1373). New York: McGraw-Hill.
Habibi, B., & Jahanbakhshi, M. (2015). Simultaneous determination of ascorbic acid, paracetamol and phenylephrine: Carbon nanotubes ceramic electrode as a renewable electrode. Anal. Bioanal. Electrochem, 7(1), 45-58.
Hasan, S. H., Othman, N. S., & Surchi, K. M. (2020). Using of Diazotized 2, 4-Dinitroaniline in Spectrophotometric Estimation of Phenylephrine Hydrochloride. Rafidain Journal of Science, 29(3), 37-46. http://dx.doi.org/10.33899/rjs.2020.166310
Ibraheem, A. A. K. (2009). Spectrophotometric Assay of phenylphrine hydrochloride Viacuopling with Diazotised p-Nitroaniline, Application toPharmaceutical Preparation. Tikret Journal of Pharmaceutical Sciences, 5(2), 182-191.
Li, K., Zhu, M., Zhang, H., & Zhao, J. (2013). Electrochemical Determination of Phenylephrine Hydrochloride Based on Graphene-TiO 2 Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci, 8, 4047-4054.
Miller, J., & Miller, J. C. (2018). Statistics and chemometrics for analytical chemistry. Pearson education.
Mzban, Q, Bahja, S, & Hassan, M.J.M. (2020). Dispersive liquid-liquid microextraction and spectrophotometric determination of cefazone and phenylephrine hydrochloride in their pure forms and pharmaceutical preparations. Plant Archives, 20(2): 6771-6777.
Nejres, A. M., & Najem, M. A. (2023). A Novel Yttrium (III) Complex for Estimating Dopamine in Pure and Pharmaceutical Dosage Forms. Biomedicine and Chemical Sciences, 2(1), 23-30. https://doi.org/10.48112/bcs.v2i1.323
Othman, N. S., & Fatah, N. T. A. (2009). Spectrophotometric Determination of Phenylephrine Hydrochloride by Coupling with Diazotized 2-Aminobenzothiazole. Rafidain journal of science, 20(4E), 69- 81.
Patel, M. N. K. (2013). Development and validation of dual wavelength method for simultaneous estimation of esomeprazole and levosulpiride in combined capsule dosage form. The International Journal of Pharmaceutical Research and Bio-Science, 2(2).
Radia, N. D., Alshamusi, Q. K. M., Sahib, I. J., Jasim, L. S., Aljeboree, A. M., & Alkaim, A. F. (2022, January). Oxidative coupling of phenylephrine hydrochloride using N, N-dimethyl-p-phenylenediamine: Stability and higher sensitivity. In AIP Conference Proceedings (Vol. 2386, No. 1, p. 030026). AIP Publishing LLC. https://doi.org/10.1063/5.0066984
Ragab, M. A., Abdel-Hay, M. H., Ahmed, H. M., & Mohyeldin, S. M. (2019). Determination of ibuprofen and phenylephrine in tablets by high-performance thin layer chromatography and in plasma by high-performance liquid chromatography with diode array detection. Journal of Chromatographic Science, 57(7), 592-599. https://doi.org/10.1093/chromsci/bmz031
Yagmur, S., Ture, M., Saglikoglu, G., Sadikoglu, M., & Yilmaz, S. E. L. A. H. A. T. T. İ. N. (2018). The quantitative detection of phenylephrine in pharmaceutical preparations and spiked human urine by voltammetry. Russian Journal of Electrochemistry, 54, 741-746. https://doi.org/10.1134/S1023193518100063
Zakaria, S. A. (2021). Simple spectrophotometric method for determination of phenylephrine hydrochloride in pure and pharmaceutical forms. Iraqi Nat. J. Chem. , 21(1): 19-29.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Biomedicine and Chemical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.


















