The Role of Gold Nanoparticles/Au-PEG-PAMAM as Drug Delivery System for Treatment of Breast Cancer
DOI:
https://doi.org/10.48112/bcs.v2i2.456Abstract
 Abstract Views: 203
Abstract Views: 203
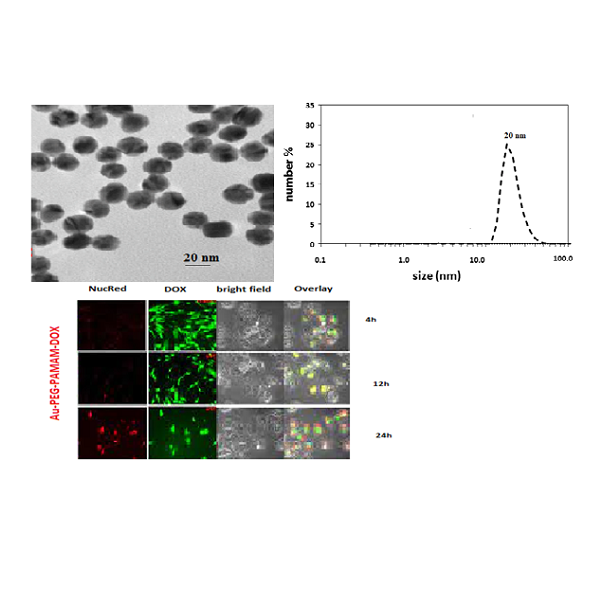
To enhance the cellular uptake and chemotherapeutic efficacy of a current chemotherapeutic medication, a nanoparticle drug carrier technology has been designed. Due to their distinctive electrical and optical characteristics, gold nanoparticles (Au NPs) have recently demonstrated intriguing medical and military uses. In the event that they come into touch with a biological system, little is known about their biocompatibility. Metallic nanoparticles have been successfully utilized for a kind of biological applications. A drug delivery system known as Au – PEG – PAMAM – DOX was produced by conjugating the dendrimer with the anti-cancer chemical doxorubicin (DOX) via an amide bond. The amount of DOX released from Au – PEG – PAMAM – DOX at a natural pH was negligible, but this amount significantly increased in an environment with a weak acidic milieu, according to studies on the release of medicines from acellular sources. A research into the intracellular release of the medication was carried out with the assistance of confocal laser scanning microscopy (CLSM). Recently conjugation to the nanosystem, in vitro viability experiments revealed an increase in the associated DOX cytotoxicity that could not be attributable to carrier components. This indicates that the effectiveness of the DOX was increased. In light of this, it has been hypothesized that the newly created pH-triggered multifunctional Au NPs- DOX nanoparticle system could pave the way for a viable platform for the intracellular delivery of a range of anticancer medicines. In the current study, the common Au NPs synthesis techniques and their well-established uses in diverse needs, particularly in biological sensing applications.
Keywords:
AuNPs, Drug Delivery, Au-PEG-PAMAM-DOX, Synthesis techniqueMetrics
References
Atun, R., Jaffray, D. A., Barton, M. B., Bray, F., Baumann, M., Vikram, B., ... & Gospodarowicz, M. (2015). Expanding global access to radiotherapy. The Lancet Oncology, 16(10), 1153-1186. https://doi.org/10.1016/S1470-2045(15)00222-3
Bentzen, S. M. (2006). Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nature Reviews Cancer, 6(9), 702-713. https://doi.org/10.1038/nrc1950
Bugno, J., Hsu, H. J., & Hong, S. (2015). Recent advances in targeted drug delivery approaches using dendritic polymers. Biomaterials science, 3(7), 1025-1034. https://doi.org/10.1039/C4BM00351A
Crooks, R. M., Zhao, M., Sun, L., Chechik, V., & Yeung, L. K. (2001). Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Accounts of chemical research, 34(3), 181-190. https://doi.org/10.1021/ar000110a
Dreaden, E. C., Austin, L. A., Mackey, M. A., & El-Sayed, M. A. (2012). Size matters: gold nanoparticles in targeted cancer drug delivery. Therapeutic delivery, 3(4), 457-478. https://doi.org/10.4155/tde.12.21
Ganta, S., Devalapally, H., Shahiwala, A., & Amiji, M. (2008). A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of controlled release, 126(3), 187-204. https://doi.org/10.1016/j.jconrel.2007.12.017
Gao, J., Huang, X., Liu, H., Zan, F., & Ren, J. (2012). Colloidal stability of gold nanoparticles modified with thiol compounds: bioconjugation and application in cancer cell imaging. Langmuir, 28(9), 4464-4471. https://doi.org/10.1021/la204289k
Golshan, M., Salami-Kalajahi, M., Roghani-Mamaqani, H., & Mohammadi, M. (2017). Poly (propylene imine) dendrimer-grafted nanocrystalline cellulose: Doxorubicin loading and release behavior. Polymer, 117, 287-294. https://doi.org/10.1016/j.polymer.2017.04.047
Gürbüz, M. U., Öztürk, K., Ertürk, A. S., Yoyen-Ermis, D., Esendagli, G., Çalış, S., & Tülü, M. (2016). Cytotoxicity and biodistribution studies on PEGylated EDA and PEG cored PAMAM dendrimers. Journal of Biomaterials science, Polymer edition, 27(16), 1645-1658. https://doi.org/10.1080/09205063.2016.1226044
He, H., Li, Y., Jia, X. R., Du, J., Ying, X., Lu, W. L., ... & Wei, Y. (2011). PEGylated Poly (amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials, 32(2), 478-487. https://doi.org/10.1016/j.biomaterials.2010.09.002
Huang, F., Watson, E., Dempsey, C., & Suh, J. (2013). Real-time particle tracking for studying intracellular trafficking of pharmaceutical nanocarriers. Cellular and Subcellular Nanotechnology: Methods and Protocols, 211-223. https://doi.org/10.1007/978-1-62703-336-7_20
Iacovita, C., Stiufiuc, R., Radu, T., Florea, A., Stiufiuc, G., Dutu, A., ... & Lucaciu, C. M. (2015). Polyethylene glycol-mediated synthesis of cubic iron oxide nanoparticles with high heating power. Nanoscale research letters, 10(1), 1-16. https://doi.org/10.1186%2Fs11671-015-1091-0
Jokerst, J. V., Lobovkina, T., Zare, R. N., & Gambhir, S. S. (2011). Nanoparticle PEGylation for imaging and therapy. Nanomedicine, 6(4), 715-728. https://doi.org/10.2217/nnm.11.19
Kurniasih, I. N., Keilitz, J., & Haag, R. (2015). Dendritic nanocarriers based on hyperbranched polymers. Chemical Society Reviews, 44(12), 4145-4164. https://doi.org/10.1039/C4CS00333K
Lane, L. A., Qian, X., Smith, A. M., & Nie, S. (2015). Physical chemistry of nanomedicine: understanding the complex behaviors of nanoparticles in vivo. Annual review of physical chemistry, 66, 521-547. https://doi.org/10.1146/annurev-physchem-040513-103718
Lazarovits, J., Chen, Y. Y., Sykes, E. A., & Chan, W. C. (2015). Nanoparticle–blood interactions: the implications on solid tumour targeting. Chemical Communications, 51(14), 2756-2767. https://doi.org/10.1039/C4CC07644C
Lee, K. Y., Wang, Y., & Nie, S. (2015). In vitro study of a pH-sensitive multifunctional doxorubicin–gold nanoparticle system: Therapeutic effect and surface enhanced Raman scattering. RSC Advances, 5(81), 65651-65659. https://doi.org/10.1039/C5RA09872F
Li, X., Takashima, M., Yuba, E., Harada, A., & Kono, K. (2014). PEGylated PAMAM dendrimer–doxorubicin conjugate-hybridized gold nanorod for combined photothermal-chemotherapy. Biomaterials, 35(24), 6576-6584. https://doi.org/10.1016/j.biomaterials.2014.04.043
Liu, L., Tang, Y., Dai, S., Kleitz, F., & Qiao, S. Z. (2016). Smart surface-enhanced Raman scattering traceable drug delivery systems. Nanoscale, 8(25), 12803-12811. https://doi.org/10.1039/C6NR03869G
Moustaoui, H., Movia, D., Dupont, N., Bouchemal, N., Casale, S., Djaker, N., ... & Spadavecchia, J. (2016). Tunable design of Gold (III)–Doxorubicin Complex–PEGylated nanocarrier. The golden doxorubicin for oncological applications. ACS applied materials & interfaces, 8(31), 19946-19957. https://doi.org/10.1021/acsami.6b07250
Rosa, S., Connolly, C., Schettino, G., Butterworth, K. T., & Prise, K. M. (2017). Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnology, 8(1), 1-25. https://doi.org/10.1186/s12645-017-0026-0
Siegel, R. L., Miller, K. D., & Jemal, A. (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68(1), 7-30. https://doi.org/10.3322/caac.21442
Tian, F., Bonnier, F., Casey, A., Shanahan, A. E., & Byrne, H. J. (2014). Surface enhanced Raman scattering with gold nanoparticles: effect of particle shape. Analytical Methods, 6(22), 9116-9123. https://doi.org/10.1039/C4AY02112F
Venkatesan, R., Pichaimani, A., Hari, K., Balasubramanian, P. K., Kulandaivel, J., & Premkumar, K. (2013). Doxorubicin conjugated gold nanorods: a sustained drug delivery carrier for improved anticancer therapy. Journal of Materials Chemistry B, 1(7), 1010-1018. https://doi.org/10.1039/C2TB00078D
Wolinsky, J. B., & Grinstaff, M. W. (2008). Therapeutic and diagnostic applications of dendrimers for cancer treatment. Advanced drug delivery reviews, 60(9), 1037-1055. https://doi.org/10.1016/j.addr.2008.02.012
Zhang, L., Zhu, S., Qian, L., Pei, Y., Qiu, Y., & Jiang, Y. (2011). RGD-modified PEG–PAMAM–DOX conjugates: In vitro and in vivo studies for glioma. European journal of pharmaceutics and biopharmaceutics, 79(2), 232-240. https://doi.org/10.1016/j.ejpb.2011.03.025
Zhang, W. L., Li, N., Huang, J., Yu, J. H., Wang, D. X., Li, Y. P., & Liu, S. Y. (2010). Gadolinium‐conjugated FA‐PEG‐PAMAM‐COOH nanoparticles as potential tumor‐targeted circulation‐prolonged macromolecular MRI contrast agents. Journal of applied polymer science, 118(3), 1805-1814. https://doi.org/10.1002/app.32494
Zhong, Y., Wang, C., Cheng, R., Cheng, L., Meng, F., Liu, Z., & Zhong, Z. (2014). cRGD-directed, NIR-responsive and robust AuNR/PEG–PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. Journal of Controlled Release, 195, 63-71. https://doi.org/10.1016/j.jconrel.2014.07.054
Zong, S., Wang, Z., Chen, H., Yang, J., & Cui, Y. (2013). Surface enhanced Raman scattering traceable and glutathione responsive nanocarrier for the intracellular drug delivery. Analytical chemistry, 85(4), 2223-2230. https://doi.org/10.1021/ac303028v
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Biomedicine and Chemical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.


















